Redox titration graph
A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction. A r edox titration is a titration of a reducing agent by an oxidizing agent or titration of an oxidizing agent by a.
9 4 Redox Titrations Chemistry Libretexts
Redox Titration Redox titration is based on the redox reaction oxidation-reduction between analyte and.

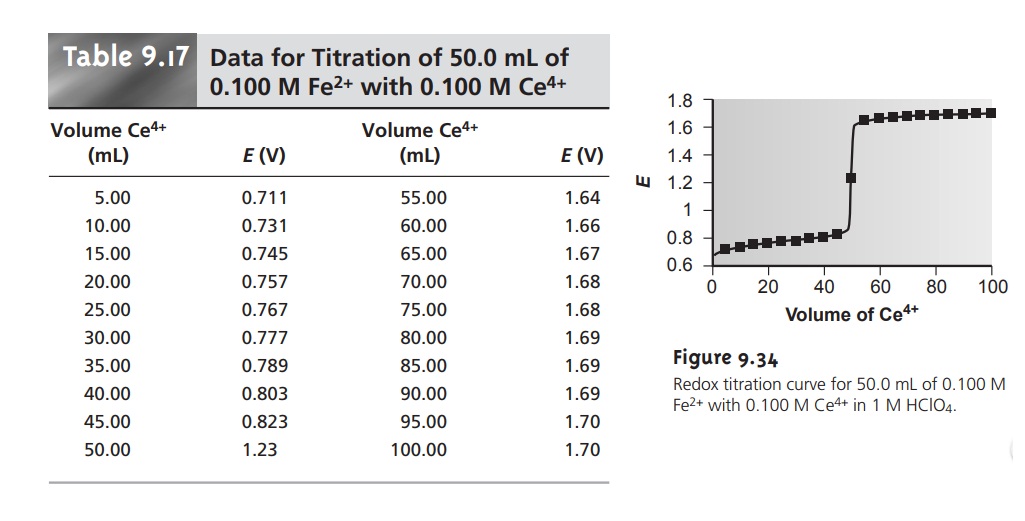
. Fe3 Ce3 E Ce 4 E Fe3 E system E In E Ce4 E Fe3 E system Equivalence Point Potentials Fe3 e-. An example of this. Redox titration is a type of titration which is based on a redox reaction between the analyte and the titrant.
Fe2 Ce4 e-. Redox titrations are based on a reduction. The Derivation of Titration Curves Titration of 5000 mL of 005000 M Fe2 with 01000 M Ce4 in a solution that is 10 M in H2SO4 at all times.
It also involves the use of a potentiometer or a redox indicator. Potentiometry is also known as Potentiometric titration. Redox Titration Curves.
Titration of a weak base with a strong acid continued Titration curves and acid-base indicators. To evaluate a redox titration we need to know the shape of its titration curve. Redox Titration Curves Fe2 Ce4.
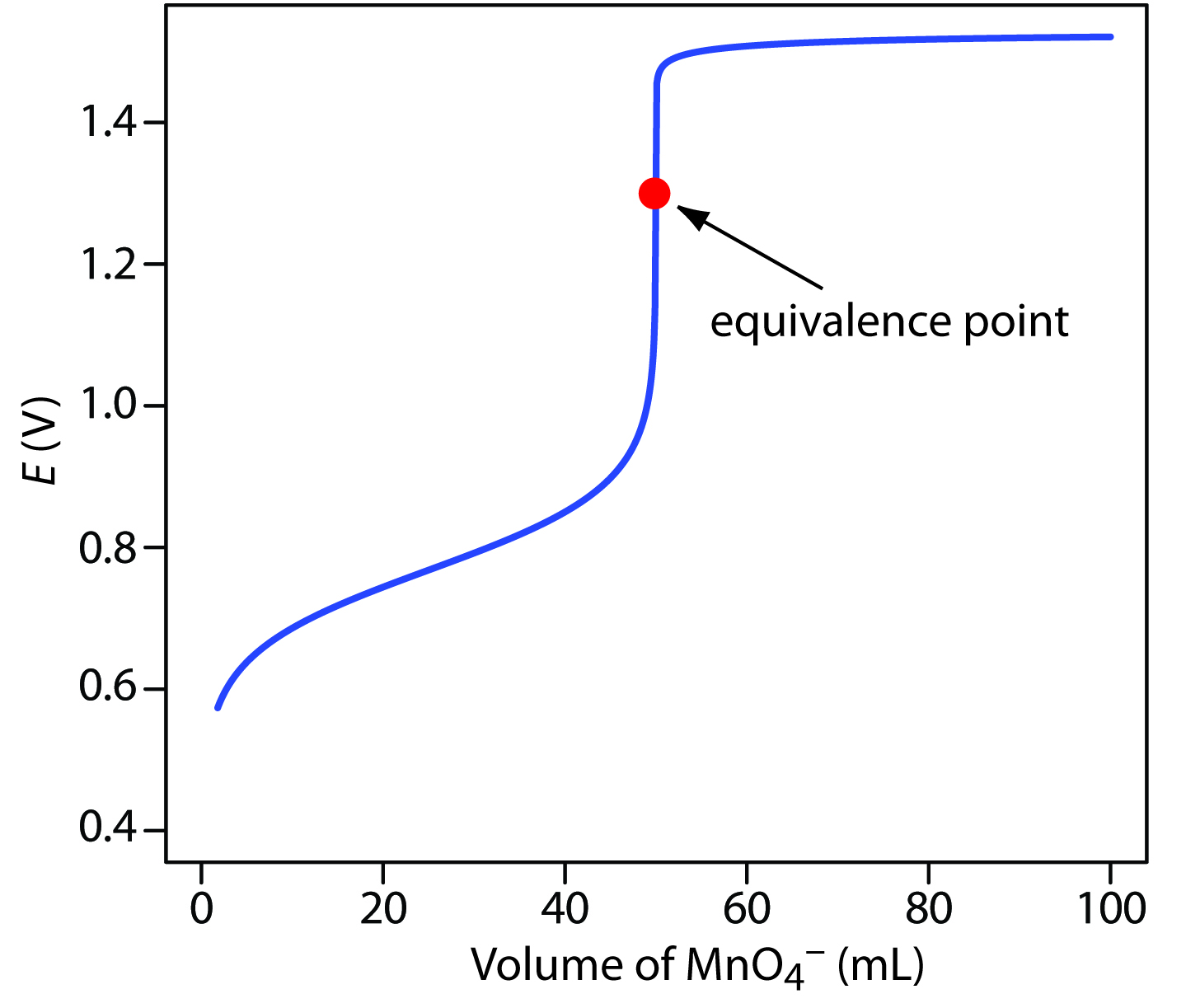
To evaluate a redox titration we need to know the shape of its titration curve. Redox Titration Curves. A redox titration curve follows the change in potential E against the volume of the titrant added.
Redox Titration Curves. 4 different types of potentiometric titrations are known. Updated on May 06 2019.
Properties of Umass Boston. A titration curve is a curve in graph the x-coordinate of which represents the volume of titrant added since the beginning of the titration. After the equivalence point has been reached there is an excess of the titrant.
Redox titration is monitored by observing the change of a electrode potential. In redox titrations an oxidizing agent is titrated with a reducing agent or vice versa. The relative positions of the inflection points and equivalence point of a homogeneous redox reaction have been studied by using the redox buffer capacity to derive an equation for.
The titration curve is a drawn by taking the value of this potential E vs the volume of the titrant. In an acidbase titration or a complexation titration the titration curve. Anne Marie Helmenstine PhD.
To evaluate a redox titration we must know the shape of its titration curve. Calculate the F from the titration of 1300 mL of 0120 M KF with 1500. The steep part of the titration curve.
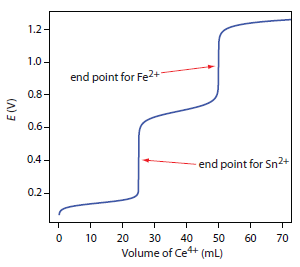
Redox titrations are used to determine unknown amounts of a substance in a solution finding the equivalence point when the titrant and analyte have reacted stoichiometrically by transferring. As in acidbase titrations the endpoint of a redox titration is. In an acidbase titration or a complexation titration the titration curve shows.
It is a technique used to characterize and measure the potential of an analyte. Ce4 e-Ce3 Ef 144V Fe3 e- Fe2. In an acidbase titration see previous unit or a complexation titration see.
Redox Titration Curves

Selecting An Indicator For A Redox Titration Image And Video Exchange Forumimage And Video Exchange Forum
9 4 Redox Titrations Chemistry Libretexts

Fig S5 Redox Titration Curves Of Fluorescence Yield Uncorrected For Download Scientific Diagram

Equivalence Points For Redox Titrations Image And Video Exchange Forumimage And Video Exchange Forum

Redox Titration Of The Cofactors Of Nar1 A Titration Curve Of The Download Scientific Diagram
2

Redox Titration Curves Of Q A In Purified Psii Particles Measured As Download Scientific Diagram

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
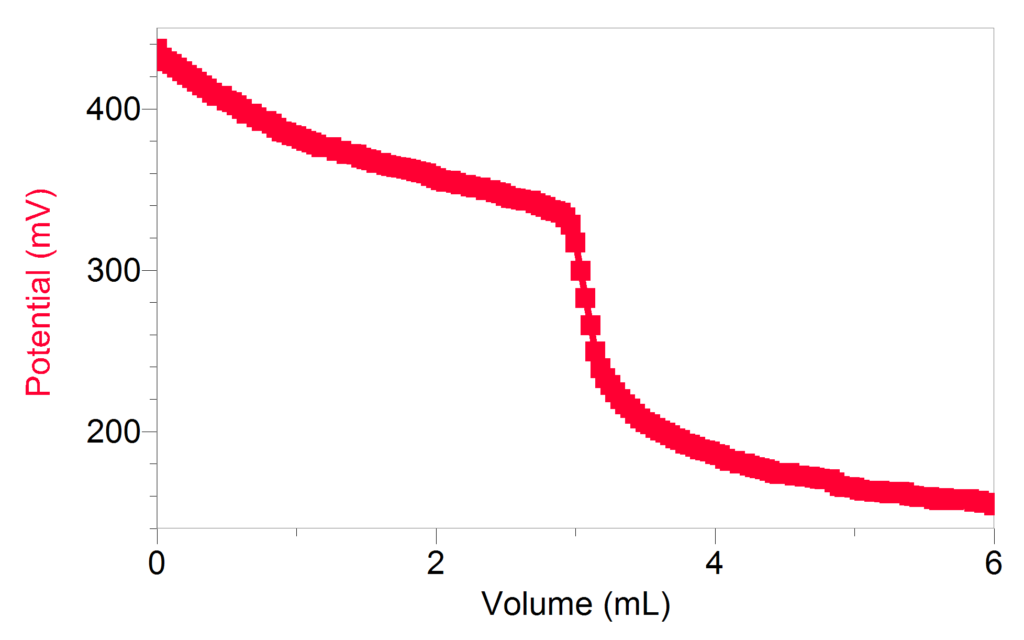
Potentiometric Titration Of Aqueous Iodine

A Redox Titration Curve Of Ferrous Ions In Solution During Reaction Of Download Scientific Diagram

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download

Redox Titration Of Various Chromatophore Membranes Reductive Dark Download Scientific Diagram

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
2
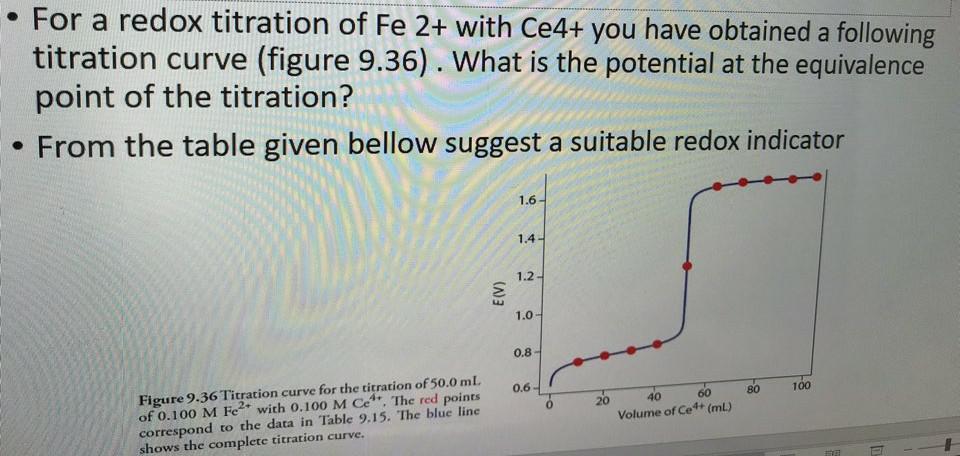
Solved For A Redox Titration Of Fe 2 With Ce4 You Have Chegg Com

9 4 Redox Titrations Chemistry Libretexts